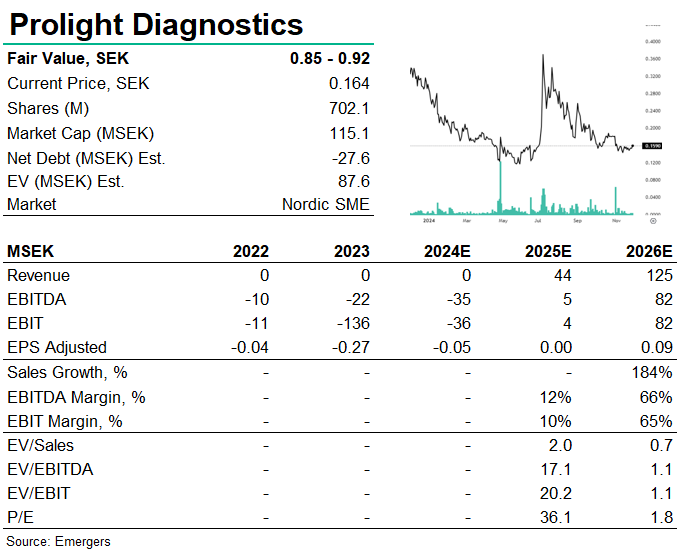
Prolight continues to make progress with its proprietary Psyros platform, with a pre-validation study, including fresh blood samples from 120 cardiac patients at St. Thomas’ Hospital in London and approximately 1,200 frozen plasma samples from biobanks. The results will be used to fine-tune the high-sensitivity troponin test and platform ahead of the clinical performance study in 2025, expected to further accelerate interest from potential partners. Prolight’s path to IVDR certification and launch in 2026 remains clear. Furthermore, a new European patent for plasma separation opens additional business opportunities. Interestingly, the exercise rate of warrants has improved from the meagre 45% for TO6 in May to 96.4% for TO7 in October, now raising SEK 12.5m. With proof-of-performance for its first-generation digital technique and the potential to expand to BNP and D-Dimer, Prolight’s prospects for commercialisation remain strong. Adjusting for the dilution, we now find support for a fair value of SEK 0.85-0.92 per share.

Johan Widmark | 2024-11-28 08:00
This commissioned research report is for informational purposes only and is to be considered marketing communication. This research report has not been prepared in accordance with legal requirements designed to promote the independence of investment research and Emergers is not subject to any prohibition on dealing ahead of the dissemination of investment research. This research does not constitute investment advice and is not a solicitation to buy shares. For more information, please refer to disclaimer.