The combination of an exclusive evaluation agreement and directed share issue to Novo Nordisk, global leader in diabetes treatment, provides both a significant validation of PharmaShell and a 63 MSEK capital injection that secures runway into 2024, thereby eliminating the risk of a previously impending rights issue.
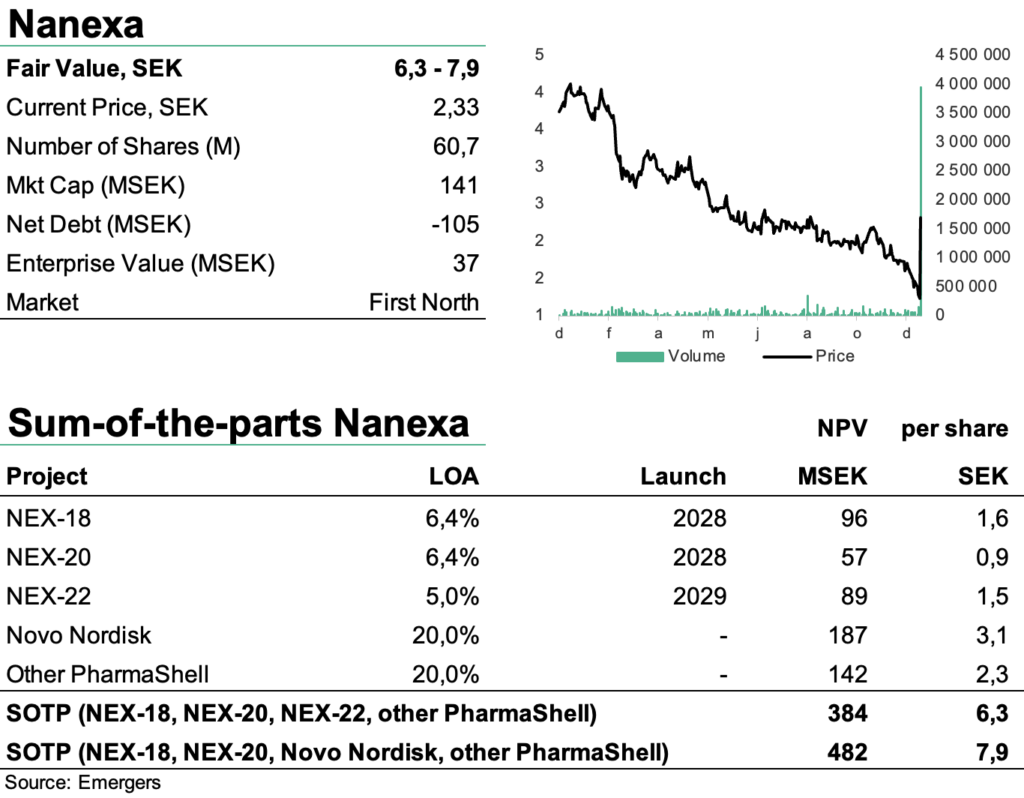
Based on rough assumptions of 20% probability for a licensing deal with Novo Nordisk, an application of PharmaShell on 10% of Novo’s portfolio long-term and a 5% royalty rate, this motivates a new fair value of 6.3-7.9 (6.6-8.1) SEK per share, after adjusting for 17% dilution and a mutual exclusivity between Novo Nordisk and NEX-22. After a +80% surge in the share price after the news, this leaves a significant revaluation potential, where we find support from a broad pipeline of activities moving into 2023.

Johan Widmark | 2022-12-23 09:00
This commissioned research report is for informational purposes only and is to be considered marketing communication. This research report has not been prepared in accordance with legal requirements designed to promote the independence of investment research and Emergers is not subject to any prohibition on dealing ahead of the dissemination of investment research. This research does not constitute investment advice and is not a solicitation to buy shares. For more information, please refer to disclaimer.
First exclusive evaluation agreement
As a Christmas gift to its tested shareholders, Nanexa has signed a Material Transfer and Feasibility Study Agreement with Novo Nordisk for the evaluation of PharmaShell on Novo Nordisk products. For this, Nanexa will receive payment of 42 MSEK in an upfront for the exclusivity and another 4.4 MSEK for work performed. The exclusivity is limited to an unspecified substance class that is narrow enough not to affect or restrict Nanexa in relation to other evaluation agreements in any meaningful way.
The deal also lets Nanexa continue the development of the proprietary project NEX-22, a long-acting formulation of liraglutide for the treatment of type 2 diabetes, which is in competition with Novo Nordisk’s own portfolio. However, should Nanexa reach a license agreement with Novo Nordisk sometime in the future, NEX-22 would most likely be either included in the deal or shut down, although that is not clear at the moment. See details in our report New project NEX-22 to add significant daily benefits for 50m patient market.
Capital injection alleviates risk of near-term rights issue
In addition, Nanexa also carries out a directed share issue to Novo Nordisk of 10m shares at a price of 1.72 SEK per share, making Novo Nordisk Nanexa’s largest shareholder at 16%. Combined with the exclusivity Nanexa will receive 59 + 4 MSEK, which along with existing cash at the end of Q3’22 at 46 MSEK, will finance all activities through 2023. Interestingly, the price at 1.72 SEK per share means a hefty 33% premium compared to the previous close at 1.23 SEK but a more balanced premium of 10% compared to 20-day VWAP.
Potential Novo license deal > current Market Cap
The deal provides a renowned, strategically important and financially strong shareholder that can bring valuable industry and sector knowledge. This means a significant validation of Nanexa’s scientific thesis and investment case.
Based on rough assumptions of 20% probability for a future license deal with Novo Nordisk, an applicability of PharmaShell on 10% to Novo Nordisk’s portfolio long term, a 5% royalty with upfront and milestones attached, and similar clinical development LOA as in the case of NEX-22, and a 15% discount rate, this corresponds to an rNPV of 187 MSEK or 3.1 SEK per share.
It is worth noting however that a potential license deal with Novo Nordisk and a continued development of NEX-22 on a stand-alone basis are most likely mutually exclusive. But it’s hard to make a meaningful scenario analysis of how that might pan out at this point, which is why of SOTP-valuation range reflect each of those scenarios.
All in all, we now find support for a fair value of SEK 6.3-7.9 per share, and with the recent start of phase I with NEX-20 (a long-acting injectable of lenalidomide for the treatment of multiple myeloma), and phase I with NEX-22 in 2023 and NEX-18 (long-acting injectable azacitidine for myelodysplastic syndrome) in 2023 or 2024, we expect an eventful year ahead.
DISCLAIMER